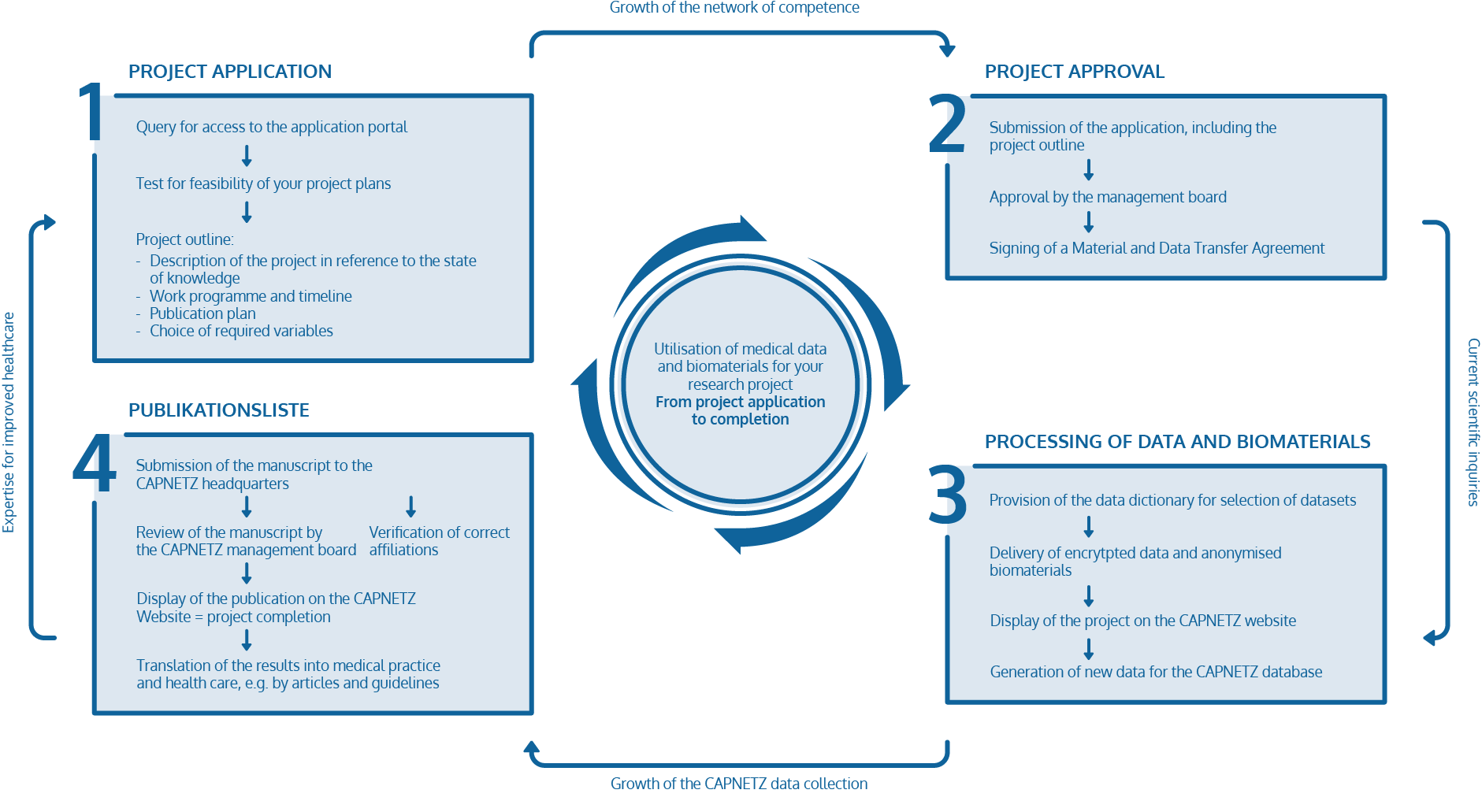
Project application
CAPNETZ STIFTUNG fosters research on community-acquired pneumonia and other lower respiratory tract infections by granting access to the CAPNETZ database and biomaterial collection.
If you have a scientific question that necessitates the analysis of clinical data or samples from CAP patients, you can apply for access. Our cohort comprises of over 14 000 CAP patients recruited over more than 20 years.
Questions?
Step 1
Step 2
Step 3
Your application is confidential. The approval is incumbent on the Management Board. Approval results in the signing of a Material and Data Transfer Agreement.
First, you will be granted access to the data dictionary for selection of your required data. Then, an encrypted data export will be provided according to your specifications and an anonymized selection of biomaterials will be sent to your lab.